Nigel Rawson | Hamilton Spectator | 17 FEB 2022
Opinions
Severely reducing drug prices by federal regulation will harm not help patients
Nigel Rawson, John Adams and Beth Vanstone | National Newswatch | 5 FEB 2022
Patients’ access to innovative medicines should have been an election issue
Nigel Rawson and Beth Vanstone | Hamilton Spectator | 9 Sept 2021
PMPRB’s Selective Use of Undefined Data Is Intended to Further its Misleading Narrative
Nigel Rawson | CHP | 15 November 2021

– Background –
The Patented Medicine Prices Review Board (PMPRB) is the federal tribunal whose role for the last 34 years has been to prevent time-limited patent monopolies for new medicines from charging “excessive” prices. In June 2016, it published a discussion document on proposed changes to its regulations, although the PMPRB leadership’s desire for revised regulations was apparent in their 2015-18 strategic plan.
Despite many criticisms to the planned changes from not only the biopharmaceutical industry but also patients, advocacy groups, physicians and other health professionals, the PMPRB and the federal government brushed them off and moved forward with the new rules. The implementation of the new regulations has been postponed three times – allegedly due to issues caused by the COVID-19 pandemic but more likely the result of evidence that the changes are ill-considered and broad-based stakeholder concern as well as several legal challenges – and is currently expected to occur in January 2022.
Throughout the past five years, the PMPRB and the federal government have maintained that the regulation changes will have little impact on the way the biopharmaceutical industry views Canada as a potential market for its products. They suggest that manufacturers will continue to perform research and development in Canada and to see Canada as a premier country in which to launch innovative drugs. This blinkered view ignores the fact that antagonistic policies in the form of rigid health technology assessments and non-transparent price negotiation processes have already reduced the industry’s interest in pursuing research, development and manufacturing in Canada. It also ignores data which have demonstrated that even the threat of the new regulations is deterring manufacturers’ interest in Canada.
– Previous Analyses –
In particular, the PMPRB has repeatedly suggested that the biopharmaceutical industry has not reduced its interest in performing research in Canada using data on newly approved clinical trials. However, the PMPRB’s analyses have failed to evaluate numbers of newly approved clinical trials by development phase.
Clinical trials of a new medicine in humans fall into three main phases designed to assess whether the medicine is safe, whether it works, and whether it is truly beneficial in real patients. Trials in the third phase are the most labour intensive and costly. They are also the most impactful on patients because they are testing medicines in patients (not healthy volunteers) with the condition for which the drug is indicated and, as such, are of direct interest to patients, particularly those with unmet health needs. Late-phase trials are commonly sponsored by manufacturers and, when performed in Canada, tend to indicate that the medicine will eventually be launched in this country. In contrast, trials sponsored by academic institutions are often earlier phase studies designed to answer esoteric scientific questions that are frequently of little direct interest or benefit to patients.
In February 2021, I demonstrated that the number of late development phase clinical trials of therapeutic and preventative medicines recorded in Health Canada’s publicly-available clinical trials database as being newly approved in the first half of each year between 2015 and 2019 was consistent (average: 135; range: 130 to 139). However, the number decreased in the first half of 2020 to 100 trials, 26% below the average over the preceding five years. A similar situation occurred in trials of oncology and non-oncology medicines, although the decrease was more apparent in non-oncology medicines. The reason for only analyzing new clinical trials approved in the first half of each year was the backlog of five to six months in the recording of trials in the database that had been previously identified by the PMPRB.
The PMPRB responded in a February 16 Twitter post suggesting that the number of late-phase trials approved in the second half of 2020 was consistent with the number in the first half. An implication of these results was that the backlog in recording trials had been eliminated in the month between the data extraction for my February 2021 article and the PMPRB’s Twitter post. However, unlike my work, the data reported by the PMPRB covered all late-phase trials regardless of whether sponsored by a biopharmaceutical company or an academic institution and, thus, failed to provide insight into any changes in the number of manufacturer-sponsored trials.
Since the recording backlog had been eliminated, I repeated my analysis using data from Health Canada’s database for each whole year between 2015 and 2020 in March 2021. Only a modest decrease of 7% occurred in the overall number of manufacturer-sponsored trials. However, between 2015 and 2020, the number of late-phase trials decreased by 23% (the decreases in oncology and non-oncology medicine trials were 15% and 25%, respectively).
Trials of COVID-19 therapies and vaccines increased the numbers in 2020. When these trials were excluded, the decrease in all manufacturer-sponsored trials between 2015 and 2020 was 12% and the reduction in late-phase trials of non-oncology medicines was 31%.
– PMPRB Latest Data –
At the 2021 Canadian Agency for Drugs and Technologies in Health (CADTH) symposium, the PMPRB presented a poster about pharmaceutical research and development investment and the number of clinical trials in Canada. This included a figure of the total number of clinical trials approved in each half year between January 2016 and June 2021 and the percentage that were manufacturer-sponsored in each time period. With these data, the PMPRB claimed that, despite a steep reduction in the number of newly approved clinical trials in the first half of 2020, the number in the second half of the year “recovered to average levels.” The implication the PMPRB wants to convey is that the threat of the new regulations is not causing a decrease in the number of new clinical trials approved in Canada.
The first point to observe in the PMPRB’s data is that the numbers of newly approved clinical trials reported are larger than the numbers recorded in the publicly-available Health Canada database for every half-year but one (Figure 1). Where these data originate is not defined; they don’t come from the first two references provided in the PMPRB’s poster but likely come from the third reference, GlobalData, a data analytics company based in the United Kingdom.
Figure 1: Newly approved clinical trials reported by the PMPRB and Health Canada by half-year
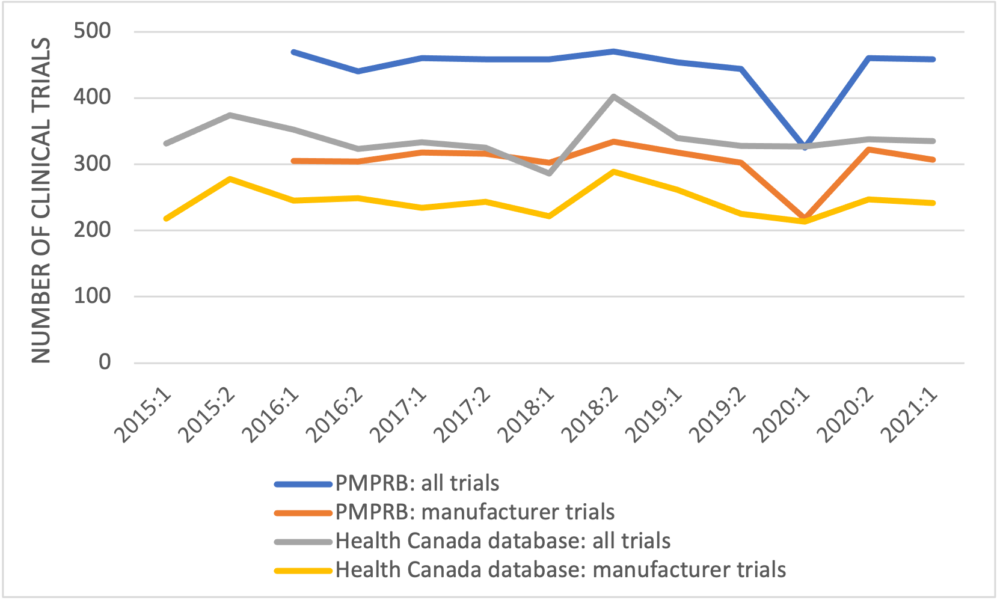
The second point is that the number of clinical trials in 2015, which is higher than the numbers in most of the later years, is excluded. Finally, the PMPRB again fails to unpack its data to examine trends in the clinical trials by development phase.
When manufacturer-sponsored trials recorded in Health Canada’s database are categorized by trial development phase (Figure 2), no trend is apparent in early-phase trials but the number of late-phase trials decreased from 168 in the second half of 2015 to 116 in the first half of 2021 – a reduction of 31%, 33% if COVID-19 late-phase trials are excluded. A similar trend was seen in the trials of non-oncology medicines with a reduction of 37% in the number of manufacturer-sponsored late-phase trials between 2015 and 2021 (45% if COVID-19 trials are excluded).
Figure 2: Newly approved clinical trials reported by Health Canada by half year and development phase
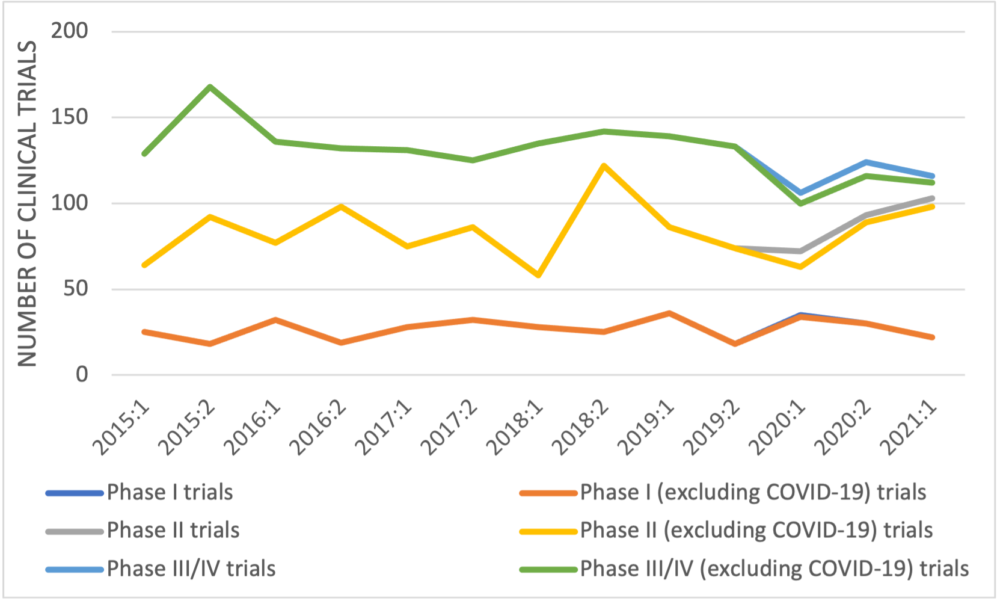
– Conclusion –
The PMPRB has used data from Health Canada’s clinical trials database in previous analyses. The data used in its CADTH symposium poster are inconsistent with information from Health Canada’s database. The PMPRB has also used GlobalData previously to suggest that not all approved clinical trials in Canada are recorded by Health Canada, although by 2019 the numbers of trials in Health Canada’s database were reasonably close to the number according to GlobalData. The question arises as to why Health Canada, the regulatory authority responsible for approving clinical trials, is not aware of all clinical trials in this country.
Whichever data source the PMPRB uses, it should provide a detailed analysis categorizing newly approved trials by sponsor type and development phase and accounting for other factors such as COVID-19 research. Simply providing overall data on newly approved trials seems designed to further the PMPRB’s misleading narrative that manufacturers are not responding negatively to its planned regulation revisions.
Information obtained from Health Canada’s publicly available database demonstrates that the PMPRB’s claim that the number of newly approved clinical trials has “recovered to average levels” is untrue with regard to manufacturer-sponsored trials. Late-phase manufacturer-sponsored trials are the costliest and the most labour intensive. They are also the ones most impactful on patients because they are testing medicines in patients with the condition for which the drug is indicated, medicines have undergone many safety assessments by this stage, and performing a late-phase trial in Canada frequently suggests that the developer will eventually launch the medicine in this country. A reduction in such trials is an indicator that the attractiveness of Canada as a country in which to launch new medicines has decreased.
Politically-instituted barriers against innovative medicines have a human toll
Nigel Rawson and Beth Vanstone | CHP Opinions | 9 Sept 2021

Photo: Beth and Madi.
In an article in the Hamilton Spectator, we wrote that patients access to innovative medicines should be an election issue. Canadians have heard little to nothing from any political party in the campaign about how they will improve access to innovative medicines that can provide effective therapy for previously untreatable diseases but whose cost is frequently beyond the average Canadian’s ability to pay.
Some politicians and academics believe that a national pharmacare program relying on federal regulations to drastically reduce drug prices will resolve patient access issues. It won’t.
Beth’s daughter, Madi, has cystic fibrosis. Her urgent need, and that of many CF sufferers, for access to Trikafta is being ignored by politicians and their officials while CADTH considers its final recommendation for the drug (its draft recommendation restricts Trikafta’s use to only a few CF sufferers) and the national price negotiator eventually accepts the drug for negotiation. CF sufferers will die as these cumbersome processes grind on.
We wanted to include Madi’s own comments in our Spectator article, but it was not possible. However, it is crucial that politicians, bureaucrats and the public understand the human toll of devastating diseases like CF, so let’s hear from Madi.
I have cystic fibrosis. It is a terminal disease that mainly attacks the lungs, but affects the entire body. When I was diagnosed, my parents were told I would be lucky to make it to 25 years old. At the age of 10, my health started declining rapidly, so we started preparing for the worst. As hard as it is to admit, I gave up. I was tired of fighting just to be alive.
But just as time was running out, a medication called Kalydeco saved my life. Kalydeco has given me 9 years of life that I couldn’t be more thankful for. I have got to experience the world, graduate high school and start college, fall in and out of love, meet the most incredible people and make the best memories. I’ve got to live.
Unfortunately, as I’ve got older, my disease has been making a slow comeback. This past year in particular has been a struggle. I feel myself slipping back, and it’s awful. Recently my lung function dropped to 53 percent – the lowest it’s been since I was 5 years old. My doctors have recently expressed that it’s crucial I switch to a new medication called Trikafta before my condition worsens. This medication corrects both defective genes in my body (Kalydeco only corrects one), but isn’t funded for all Canadian CF patients that could benefit.
Gaining access to Trikafta is a huge fight for Canadians. Families and patients have been working non-stop to get this medication to those who need it. As I have been struggling silently, I’ve been watching the CF community come together and fight for what is right.
I just want to thank everyone who has contributed to this fight for Trikafta. You are all absolutely amazing and I can’t thank you enough for the work you have been doing to save not only my life but my friends’ lives. To my fellow CF’ers fighting, you ROCK. It’s exhausting fighting for your life and fighting the government at the same time. You are all amazing!
We are close to gaining Trikafta. I can’t wait to celebrate with all of you when this fight is finally over. I promise to do what I can to help finish this once and for all. Stay strong and keep fighting!
Unless you have ice in your veins, it is impossible to read Madi’s words without being moved, firstly, by her strength to keep fighting both CF and the system and, secondly, by the suffering caused by a disease that could be helped by access to an innovative medicine.
The suffering is not confined to the patient but extends to their family and friends. Her mother describes the impact and the gruelling fight against both CF and governments eloquently.
Fighting chronic illness is a life-changing experience not only for the patient but the entire family. The daily challenges of appointments, therapies and hospitalizations, along with investing time and energy into supporting efforts to raise money and participate in research to improve treatments and search for cures, is more than many patients and families can endure.
To invest personal resources in an effort for better outcomes for patients and then have to battle the government to access these life-changing, life-saving medications is inhumane. We have spent Madi’s entire life doing therapy, attending clinics, and enduring hospitalizations trying to save her.
The first CF modulator (Kalydeco) was the culmination of decades of fundraising and research, and it represented hope for CF patients. We had no idea that it would unlock the door to an unforeseen battle with our own government. We have been advocating for nearly a decade now, over half of Madi’s life, to access these medications. During the past decade, we have only seen changes that make the process nearly impossible for Canadian patients trying to access these medications.
As we watch countries around the world create solutions to make these innovative medications available for patients, Canada has thrown up additional roadblocks to ultimately prevent access to the majority of these medicines. As a parent and advocate, I can only see a future where, rather than being able to celebrate innovation and the benefits to patients, we will forever be embroiled in a battle for new and improved therapies.
It shouldn’t be this hard. Canada needs to invest resources in finding a fast and effective pathway to these medications.
When politicians and academics suggest that national pharmacare will improve access, you should realize that they haven’t a clue about the real toll of their obstacles to drug access on human health. A pharmacare program covering only a small number of medicines for common disorders, with a vague promise to phase in other medications over an unspecified number of years subject to regulations forcing severely reduced drug prices to make the program affordable, is not going to help individuals like Madi – it’s just false hope.
Patients whose health could be improved by innovative medicines will continue to suffer and die prematurely if Canada’s hostile attitude towards drug developers is maintained. Canadians need a federal government that adopts a collaborative relationship with the biopharmaceutical industry to ensure patients have access to new therapeutic advances now – and into the future.
Nigel Rawson is an independent researcher and an affiliate scholar with the Canadian Health Policy Institute. Beth Vanstone is a long-time advocate for Canadians with cystic fibrosis and a director with CF Get Loud. The views expressed are the authors’ own and do not necessarily represent those of organizations with which they collaborate.
Appeal court exposes PMPRB abuse of power and bias on drug pricing
Nigel Rawson and John Adams | Financial Post | August 18, 2021
We no longer need the PMPRB
Brett Skinner and Nigel Rawson | The Hamilton Spectator | 13 JUL 2021
Yanick Labrie testifies before parliamentary committee regarding pending PMPRB regulatory changes
Yanick Labrie | HESA | 11 June 2021
Time for the PMPRB to go
Nigel Rawson and Brett Skinner | The Hill Times | June 17, 2021
Worrying Signs about Private-Sector Investment in Clinical Trials
Nigel Rawson, PhD | National Newswatch | April 30, 2021
PMPRB misguided: Evidence warns severe price controls will reduce availability of new medicines
Brett Skinner | CHP | 1 May 2020
While the country is focused on the COVID-19 pandemic, Canada’s federal government is quietly introducing new drug price controls that threaten to cause shortages of innovative medicines for Canadians.
Since 1987, the prices of newly developed drugs have been regulated by the Patented Medicine Prices Review Board (PMPRB). The Patent Act gives the Board power to control the prices of patented drugs according to its own guidelines. S. 83 (1) states that if a patented medicine is being sold at a price that, “in the Board’s opinion” is too high, the Board may order the price to be reduced “to such level as the Board considers not to be excessive.”
In July, the Board will launch an unprecedented experiment with price controls that are more complex and extreme than any other regulatory regime in the world. The draft guidelines published by the PMPRB include changes to the countries used for international price references. The Board is also introducing controversial pharmaco-economic factors to set drug prices. In addition, the Board will regulate not just manufacturers list prices, but also the net prices that are normally determined through rebate negotiations between manufacturers and public drug plans or private insurers.
The PMPRB estimated that the combined changes would reduce the prices of new medicines by more than half. Research conducted by Nigel Rawson and published by the Canadian Health Policy Institute (CHPI) has shown that the changes will reduce the maximum list prices for innovative medicines by as much as 84 percent from current levels.
Pharmaceutical manufacturers warn that such arbitrary and severe price cuts will discourage companies from launching new drugs in Canada and will thereby reduce the availability of important new therapies. The Board disagrees. In February, PMPRB Executive Director Doug Clark told Reuters there is no evidence that regulating drastically lower prices will reduce access to new drugs.
However, a 2019 study by Kanavos and colleagues at the London School of Economics and published in the European Journal of Health Economics showed that manufacturers delay launching products in countries with highly regulated prices. This led to reduced availability of medicines in those countries.
Earlier research by Danzon and colleagues at the University of Pennsylvania and published in the journal Health Economics in 2004, analyzed the effect of price on the launch of new drugs in 25 countries. The study found that manufacturers delay launching in countries where regulation reduces prices below levels expected from local market characteristics.
In 2018 CHPI published a study I conducted using data from the PMPRB and the OECD to see if the number of new drug launches was affected by the market price level, controlling for differences in per capita GDP and population across 31 countries. Market price level was the only variable that was a statistically significant predictor of the number of new drug launches.
The pending regulations may already be discouraging pharmaceutical companies from launching new therapies in Canada. In a 2020 study published by CHPI, Rawson examined changes in the availability of new drugs since the PMPRB first announced its intention to revise the guidelines in 2016. The percentage of new drugs available in Canada within a year after becoming available in the United States decreased from 55 percent in 2016 to less than 16 percent in 2019. The results suggest that the pharmaceutical industry has taken note of the impending changes and begun to delay launching new medicines in Canada.
The government reiterated its commitment to “making evidence-based decisions” in Health Canada’s 2019-20 departmental plan. Yet the PMPRB has ignored evidence that contradicts its price control agenda. By allowing this regulation to come into effect, the government is making a huge mistake that will have significant consequences. It will discourage pharmaceutical companies from launching drugs in Canada. Canadian patients will wait years to access the same new medicines that are immediately available to Americans.
Think the vaccine shortage is bad? Wait until you see what price regulations do to our drug supply
Brett Skinner | Financial Post | March 9, 2021